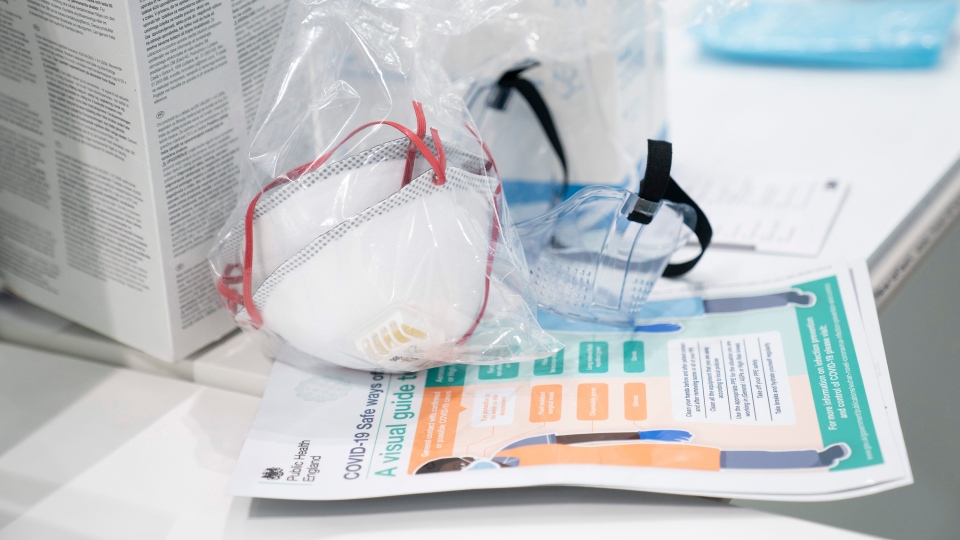
LONDON, ONT. -- At the beginning of the COVID-19 pandemic many manufacturing companies shifted their operations to begin making personal protective equipment (PPE) for hospitals.
While creating face-coverings for public consumption is encouraged, the head of the Canadian Federation of Nurses Union (CFNU) says not all PPE is certified for front-line health care workers’ use.
Linda Silas, president of CFNU, says her inbox is flooded with different manufacturing companies offering PPE without a Health Canada stamp of approval.
“We’ve seen some (PPE) that have a funny smell, the texture is different…they were all returned.”
Silas says PPE has to meet what health-care workers call the ‘NIOSH standard,' National Institute of Occupational Safety and Health set by Health Canada.
“They are not the masks that you sell in stores, they are really specified for the health care workplace...We have had situations where they were not (NIOSH approved) and I guarantee health care workers sent them back.”
Health Canada requires that NIOSH-approved masks are labelled with the performance standard, the corresponding designation (for example N95) and meet all aspects of the performance standard, including labelling
If neither of the above are present, the product must state that it is not NIOSH-approved.
Under occupational health and safety laws, it's up to the employer to make sure employees have the appropriate safeguards and equipment necessary in the workplace.
Silas says that in the provinces in Canada where this occurrence happened, employers have worked very quickly to take the inadequate PPE off the shelves.
“It’s really not up to us to go to websites to make sure (PPE) is approved or not. When you have it in your hands, if there is not the certified approval you go back to your employer. We have to bring the responsibility back to the employer.”
It's not just face-masks - gowns, gloves and face shields also have to meet Health Canada’s standard for hospital use.
Silas says most face shields that are seen in stores across the region can not be used in hospitals.
They too have to have the proper certification. Even then, they cannot be used as a stand-alone product, but only in accordance with an N95 or NIOSH-approved mask.
Western University’s Faculty of Engineering went through the certification process when they created face shields at the beginning of the pandemic, in partnership with General Dynamics Land Systems.
“We had input from medical doctors, practitioners, they looked it over for weeks,” says Peter White, executive director for Strategic Partnerships at Western University.
White says the face shields were adapted many times before they applied for Health Canada certification.
“It is a long process, something that has to be planned and worked through, you have a series of applications...Health Canada had some changes to our design before they were approved and used in facilities.”
Western University’s certified face shields have gone to over 50 institutions and hospitals across London, Toronto, Ottawa and Indigenous health services in southwestern Ontario.
Silas continues to thank and encourage companies to make PPE for hospitals and consumer use, but adds it's important for companies to work with the government to get the Health Canada approval so they can be used in health-care settings.
“We’ve seen many Canadian companies taking a lead and making partnerships and we need that...but PPE needs protection and they have to be approved by the province then given to employers.”