TORONTO -- Ontario will start providing interim funding for a costly drug used to treated a rare genetic condition, but a small group of sufferers from the disease is urging the province to make that permanent.
An advocacy group estimates the drug, Soliris, could help about 30 people in Ontario who have atypical hemolytic uremic syndrome, a life-threatening condition in which the immune system attacks the body's cells damaging blood vessels and can lead to heart attack, stroke and kidney failure.
Health Ministry staff were not able to provide the cost for Soliris, but noted that reports have pegged it at between $500,000 and $700,000 annually per patient.
Health Minister Eric Hoskins announced Wednesday morning that the executive officer of the province's public drugs program informed him earlier this week that Ontario will provide interim funding for Soliris to aHUS patients "who meet defined clinical criteria of the disease."
Progressive Conservative health critic Christine Elliott slammed the minister's "last-minute, half-baked announcement," published minutes after aHUS Canada held a news conference at the legislature.
"How convenient is it that the announcement was made today," she said in the legislature. "I guess it takes a little bit of the heat off you, but I think people need to know that this is not going to be funded for all aHUS patients, it's only a one-off strategy. Only those who are really, really sick are going to be able to get access to this, not everybody who needs it."
Hoskins said the pan-Canadian process looking at the drug had indicated there wasn't sufficient clinical evidence to merit funding. But while those at the national level are reviewing additional information, it was important to step in and provide interim funding, Hoskins said.
Elliott said the patients are not satisfied.
"There's no full funding that's been promised for them on a permanent basis," she said after question period.
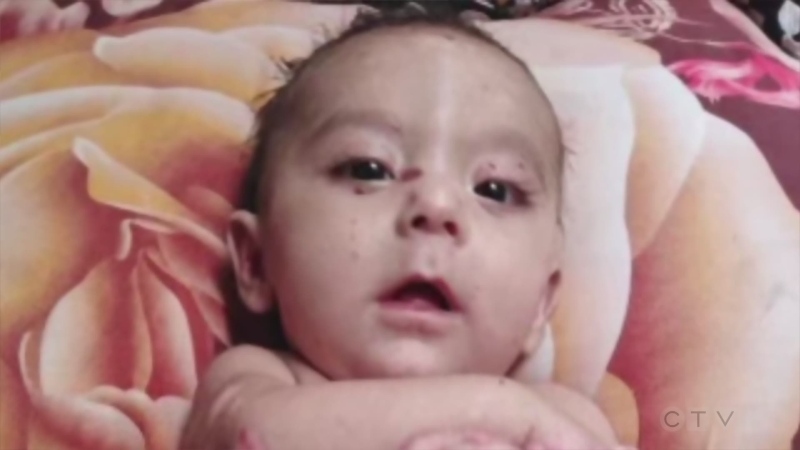
Sonia Girotto's then-10-year-old son, Joshua, spent five weeks in hospital three years ago, with internal bleeding, kidney failure, a blood clot and underwent a kidney biopsy.
"He reached a point within his first week where he didn't recognize us, couldn't speak or hold his head up," she said.
Joshua's doctor entered him into a clinical study for Soliris and soon after his kidney function was fully restored and his condition now has little impact on his day-to-day life, Girotto said.
"Today Joshua has a paper route, he plays soccer and the drums," she said. "For our family, access to Soliris meant that we got our little boy back, and most importantly Joshua got his life back. Now we want the same for everyone who lives with this devastating disease."
Girotto said she knows the cost is high, but permanent funding is urgently needed.
"I think that the patient population group is tiny, so what it (adds) to the overall budget is not insignificant, but very small and I think it's worth it," she said.