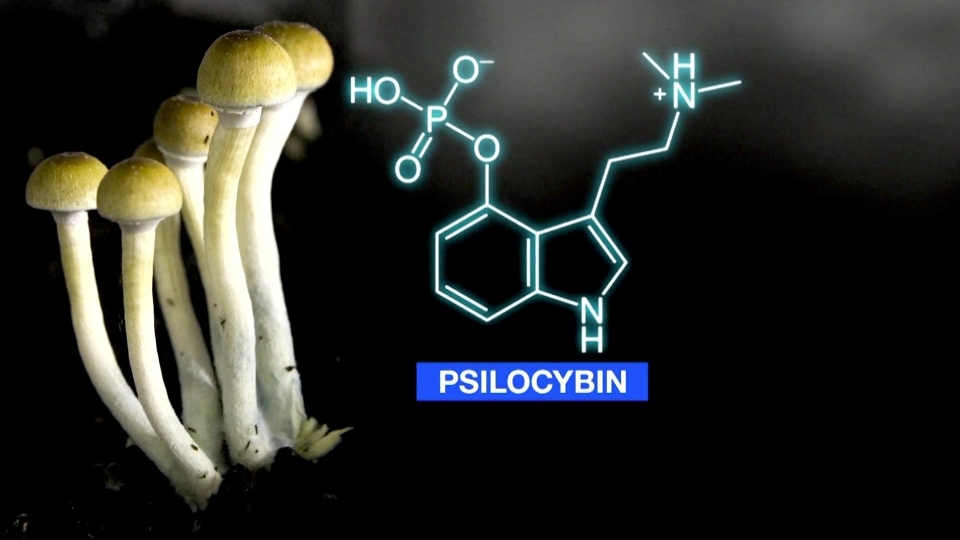
LONDON, ONT. -- New Leaf Canada has received approval from Health Canada that will allow its subsidiary Psirenity to begin construction of a psilocybin research facility in Norfolk County.
The new research centre will be roughly 185 square metres within the larger New Leaf facility. Its approval was announced last week.
There is growing evidence that psilocybin could be used to treat anxiety and depression.
“The process of attaining the CDSA (Controlled Drugs and Substances Act), the dealer's licence, which allows us to work with controlled substances such as psilocybin. We began the application almost a year ago,” says Psirenity CEO Chris McCullough.
With the approval the company will focus its research and development into these key areas:
- formulation of investigational products for future clinical trials
- supply of investigational products for Psirenity's clinical trials
- psilocybin and psilocin production
- psilocybin containing mushroom strain development
- supply of prescription-controlled drugs for Psirenity's Health and Wellness Therapy Clinics
- extraction and isolation of psilocybin and psilocin method development
“What we’re working on is a micro-dosing platform. Something that doesn’t have the psychotropic effects where you can take your micro-dose and be able to go about your day. You can go to work, you can drive your car and so forth.”
McCullough says they are working with regulators and Health Canada to work toward new treatments in psychedelic-enhanced mental health treatments.
Currently, Health Canada has not approved any treatments for use, and psilocybin is considered an illegal substance under the law. However, there have been exceptions made on compassionate grounds in some cases.
“As an emerging treatment mechanism, especially for mental health and wellness, I think there has to be safeguards put in place, there has to be a frame work by the governing bodies,” McCullough explains.
The company became the first to be approved by the University of the West Indies ethics community to conduct a phase one clinical trial for safety and efficacy around micro-dosing.
They also established a partnership in Jamaica to run a similar trial that will conclude prior to the start of a physician-led Canadian study tentatively scheduled to begin at the end of 2021 or in early 2022.
“We will have completed by that time our phase one clinical trial in Jamaica, which will also provide the necessary supporting data and results to help bolster the physician-led trial in Canada,” says McCullough.
Construction on the facility in Norfolk County is expected to begin within 60 days.