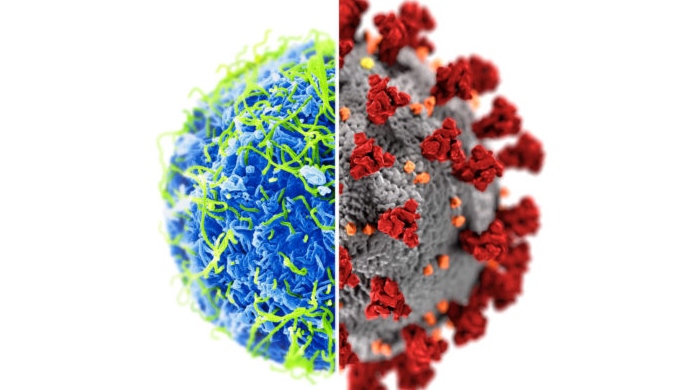
LONDON, ONT. -- Western University says the use of Ebola emergency vaccines provides an ethical blueprint for the COVID-19 response.
Despite not having safety and efficacy data from the final phase of clinical trials, countries such as China and Russia have already started vaccinating against COVID-19.
The move has been widely criticized for prematurely exposing people to these unlicensed vaccines.
However, Western University professor and member of the WHO’s Ethics and COVID-19 Working Group, Maxwell J. Smith says the move to push vaccines quicker is not unprecedented.
Countries can issue emergency-use authorizations for unlicensed vaccines during public health emergencies.
Smith says it’s something Canada shouldn’t rule out, but rather take a more transparent approach
“We have to be very transparent about how emergency-use authorizations are done,” Smith said in a statement. “We need to make sure decisions are based on favourable risk-benefit ratios.”
Emergency-use authorizations are a regulatory mechanism enabling the public to gain access to promising investigational drugs, devices and vaccines when they have not yet received regulatory approval and licensing.
“COVID-19 is a public health emergency of international concern. And it’s going to need a novel vaccine so it's pretty similar to the Ebola situation in Africa so we should learn from it,” Smith added.
“The other thing is that Guinea and the Democratic Republic of the Congo, which ultimately provided access to unlicensed vaccines, did so in a very transparent and coordinated fashion with the World Health Organization. That stands in stark contrast to what Russia and China have done with their COVID-19 vaccines.”
Smith says that even if China and Russia COVID-19 vaccines are safe and effective, without transparency and rigorous ethical oversight the public may not trust them.